AI and Electronics for Medicine 2024
»Rising as Hell«: The Demand for Medical Chips is Growing Rapidly
Does medical technology need specific semiconductors? If so, how many? The chip session at the AIEFM Conference 2024 in Dresden shows how the digitalization of medicine is changing the potential and requirements - and predicts a rapidly growing sales market similar to the automotive industry.
»Medical technology is ten to 20 years behind in chip technology«. It was a bitter truth with which Prof. Dr. med. Jochen Hampe from TU Dresden began his presentation »Medical Need for Dedicated Medical Semiconductors« in the session on medical semiconductors on day three of the AIEFM Conference 2024.
However, the pressure to digitize is great and many digital health products, some with artificial intelligence, have long been on the market: According to Hampe, the further networking of healthcare urgently requires dedicated medical microsystems that combine sensors, AI-capable data processing, medically safe connectivity and dedicated medtech functions on one chip. If the small size of the market and overly stringent requirements were previously a barrier to entry for expensive development, these are no longer an issue according to Prof. Hampe.
»Digital medical electronics are booming. Medical-specific semiconductors can be used in various devices across manufacturers,« says Hampe, who compares medical technology to the beginnings of automotive semiconductors and vehicle digitalization. »Processes and procedures can be transferred from the automotive industry, and we see a large and rapidly growing market potential.« For the co-project coordinator of the future cluster Semeco (Secure Medical Microsystems and Communications), which is funded by the BMfBF, this is compounded by the need for miniaturization in medical technology and the trend towards »single use« devices.
The Market for Medtech Chips is set to Explode
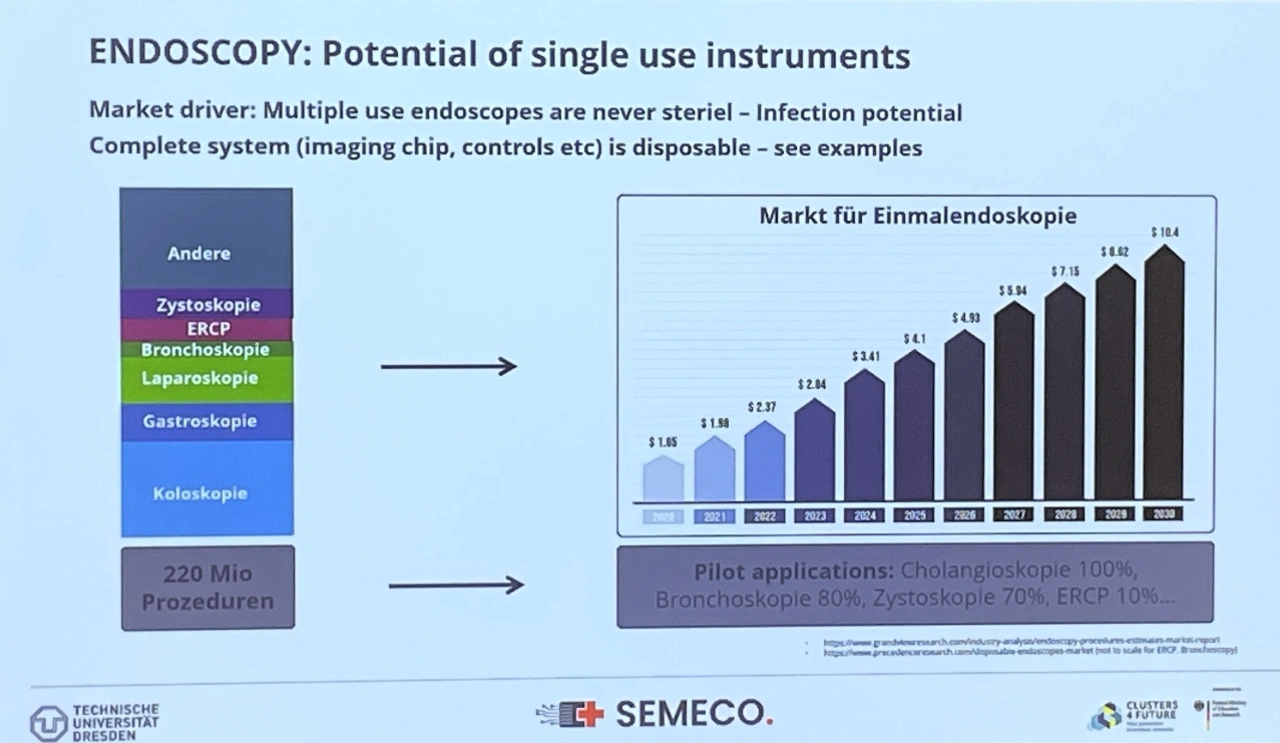
Currently, 220 million minimally invasive procedures are carried out each year with multi-use endoscopes alone, and growth rates in emerging countries are up to 10 percent per year. Even if all hygiene measures are carried out correctly, there is always a residual risk in terms of sterility. The world is demanding single-use endoscopes, and the market is expected to quadruple to 10.4 billion US dollars by 2030 (Fig. 1). The number of embedded vision systems required, including the necessary chips, will increase accordingly by leaps and bounds and provide a technological boost with new assistance functions that can then be implemented.
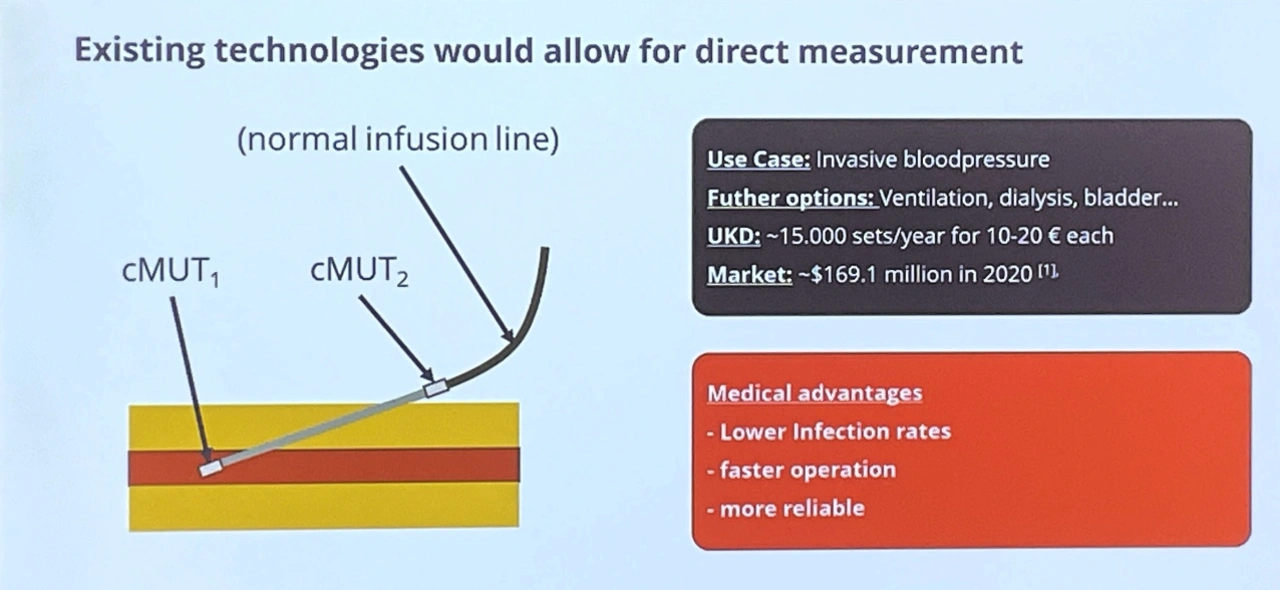
Prof. Hampe cites the gradual equipping of consumables for medical fluids with electronic measuring and monitoring functions as a further market driver. In future, millions or even billions of infusion bags, syringes, dialysis or ventilation tubes will be able to detect the flow of fluids and their properties directly on the patient, with a low risk of infection and greater reliability (Fig. 2).
While medtech manufacturers today still mostly develop the medically safe chips urgently needed for intelligent medical products from a single source and produce them according to their individual requirements, Hampe's goal - from a technical and regulatory perspective - must be modular medical technology development and certification: led by medical technology manufacturers who then rely on suppliers for modular sub-systems, complex components and, as before, standard components for individual assembly.
Biotronik: Proprietary ASICs for Pacemakers
One of these monolithically developing medical technology companies is Biotronik - for good reason. In his presentation »Miniaturization for Implantable Medical Devices Requires Custom Semiconductors«, Thomas Dörr, Head of Research and Development at the leading manufacturer of pacemakers and cardiological implants, used the development of heart implants to show how much the technological development of ever smaller and, above all, safer pacemakers is dependent on dedicated, in the case of Berlin, proprietary chipsets.
Critical components and ASICs in particular have been developed and manufactured in-house by Biotronik since the early 1980s. While a pacemaker still stood next to the bed in the 1960s, the most important criteria, according to Dörr, are still the miniaturization of the devices as well as hardware acceleration, state-of-the-art cybersecurity, always-on connectivity and longevity thanks to reliable battery life. Whereas a data record used to be read out once a day, today it is all about permanent device monitoring and remote device management, including firmware updates. In addition, clinical workflows constantly require new functions. By comparing a pacemaker from 2006 with the current model for 2026, Dörr shows that Moore's Law still applies in medicine.
| 2006: Lumax 340 DR-T | 2026: Acticor 7 VR-T DX | |
|---|---|---|
| Volume (cc) | 35 | 30 |
| Shock energy (J) | 28 | 40 |
| Service life in years | 7,5 | 15 |
| Connection | Remote Monitoring | Remote Programming |
| Analog | 180nm | 55nm analog/digital/780kB, 2x 32bit CPUs |
| Digital | 130nm, 8bit CPU | |
| Radio | 130nm MICS band | 130nm MICS + Cybersecurity |
| Memory | 512kB SRAM | 128MB EEPROM |
Advanced miniaturization now even allows for connection-free pacemakers that can be implanted directly into the heart with a superior voltage. Although the transmission-free mini devices do not offer monitoring, the data can be read out. The Head of R&D sees AI functionality IN the devices as the next step, and an FDA guideline has already been issued. »In-memory processing will lead to implant-specific AI accelerators in order to further reduce power consumption,« says Dörr. In addition to miniaturization, the integration of far-reaching embedded AI functions is another urgent reason for tailor-made semiconductors for medical implants.
NXP: Low Latency, Low Power Consumption
Miniaturization was also cited by Henning Möller from NXP Semiconductors in his presentation »The Case for a Dedicated Medical Chip Portfolio« as the first reason for individual semiconductor development tailored to medical technology requirements. Using the example of a hearing aid, he showed how always-on operation, new connection modes such as ear-to-ear, TV streaming and control via smartphone app in combination with higher audio performance through AI-supported real-time processing can become a game changer. On the other hand, it is precisely these functions that place the highest demands on hardware development with latencies of less than 5 milliseconds and extremely low power consumption.
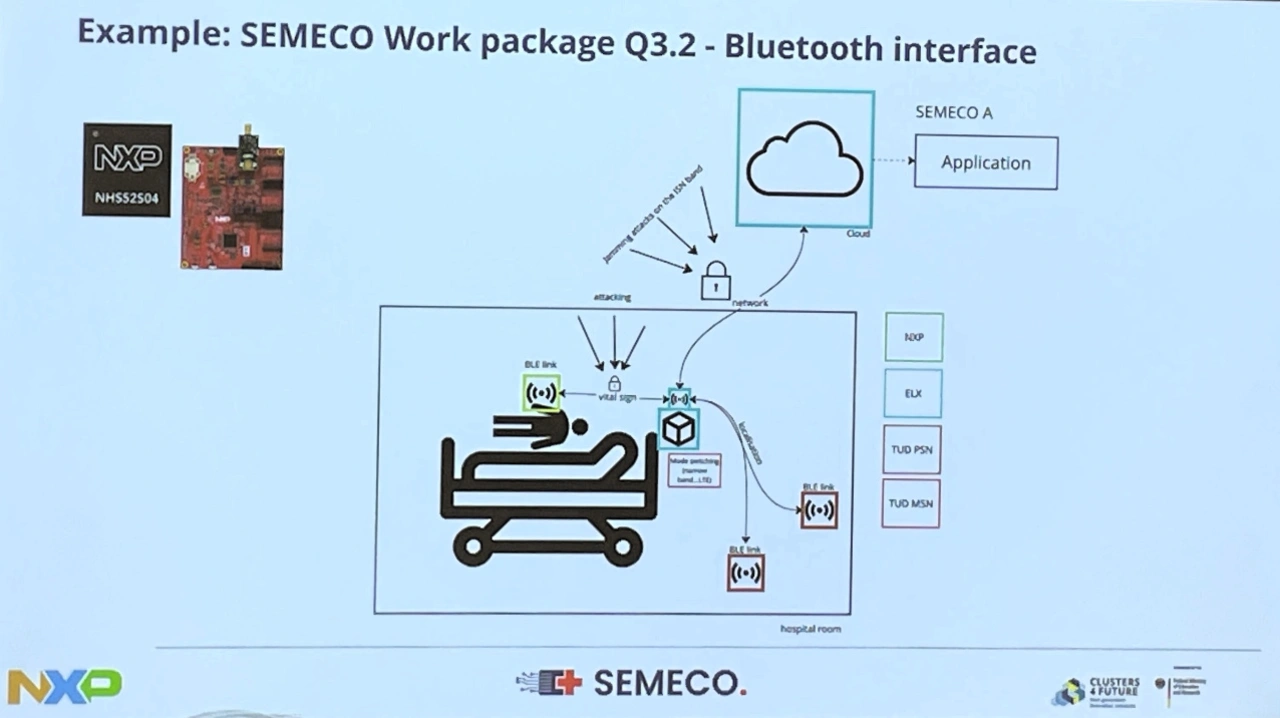
As a second example, the Semeco project partner presented a Bluetooth LE chip for blood glucose measurement (Semeco work package Q3.2), which uses Bluetooth Low Energy to communicate with the hospital network and/or cloud from the moment of admission and makes the patient's vital data permanently monitorable during their hospital stay via »smart and cool ICs« (Fig. 3).
Clinical Monitoring Taken a Step Further
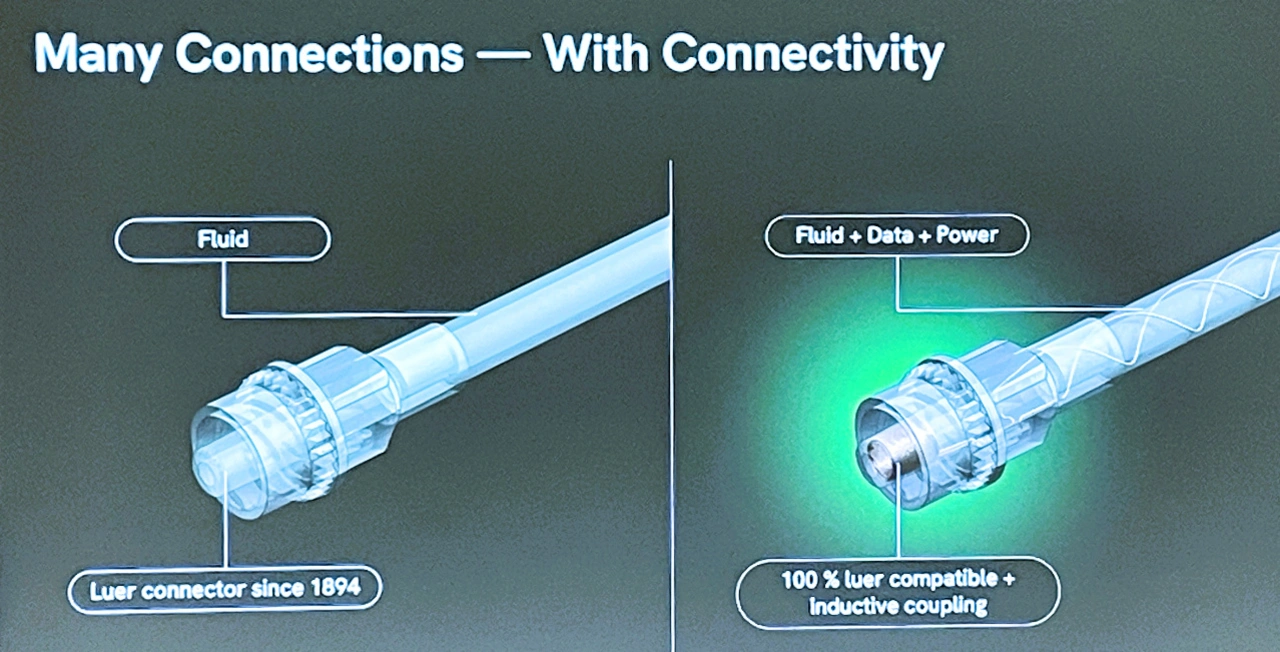
The practical example of NXP could be seen as a direct transition to Dr. Nora Herzog: As medical director of the Dresden start-up ConnCons, she showed in her presentation »Clinical Consumables as mIoT Devices« how important the translation of »all the fancy technology« into concrete clinical applications is. More patients, more treatments and also a longer (survival) lifespan would require fundamental automation directly at the hospital bedside. She used a digitized infection therapy via a smart Luer connection to show how easy this can be. The standardized connection system for infusions and injections has been in use since 1894 and is now becoming digital without any loss of functionality (Fig. 4).
Using an RFID chip in the medical bag and a data and power conductor integrated into the tube, the pump knows all the settings and criteria and adapts the fluid control to the individual patient. With the right planning software, the »smart consumable« could be used to create a closed circuit in oncology, for example, according to Herzog. The resulting »cloud-to-point-of-care« ecosystem also enables AI connection and makes work much easier for nurses and carers.

Currently, according to the Folfox scheme, the medication bag has to be set up, changed or checked four times and the patient has to wait an average of 74 minutes. With smart infusion bags (Fig. 5), only one setup would be necessary and the waiting time for cancer patients would be eliminated to zero. Overall, this would result in a reduction in administration time of 32 percent and personnel costs would fall by 67 percent per treatment. Dr. Herzog sees great potential for dedicated medical chips due to the large number of potential applications: »Tailor-made medtech chips could solve many medical applications in one fell swoop«.

Wearables and Consumables Drive Demand
The subsequent panel discussion (Fig. 6) first looked at the reasons why innovative medical devices, such as wearable technologies and electronically upgraded and networked consumer products, have not been on the market for ten or 15 years - the technology is already available. The participants in the discussion identified several obstacles which, in combination, contribute to the technological lag compared to consumer products or the automotive industry: One key problem highlighted by Prof. Hampe is the shortcomings in the medical system, in particular the lack of knowledge among doctors about the technical details of devices such as pacemakers. In addition, regulatory challenges and the inertia of the medical device industry are seen as obstacles. Uwe Gäbler from Infineon and Henning Möller from NXP, who had recently joined the panel, emphasized the need for better networking between doctors and technicians in order to understand the actual needs in practice.
Another point is the market dynamics. Thomas Dörr from Biotronik used the example of pacemakers to clearly point out that the market for medical chips is currently smaller compared to other sectors such as the smartphone industry, which makes it more difficult for chip manufacturers to enter the market - even though many semiconductor giants are currently positioning themselves in the healthcare sector due to the rapidly growing demand, in Dörr's own words: »rising as hell«. Nevertheless, the medical technology market with its very specific requirements for reliability, low voltages, long development and life cycles as well as the necessarily excessive testing and certification remains an expensive and difficult market environment. Even if, for example, Infineon believes that qualification cycles are being shortened by a factor of 3 or 4 because customers are simply demanding this. Nora Herzog added that there is a lack of structures in the healthcare system that would enable process optimization, which often leads to a different mindset than in project work.
The discussion revealed a trend from a consumer perspective that is influencing and significantly driving medical technology. Consumers are increasingly turning to wearables and other medical-related monitoring devices for vital data monitoring - »which are not necessarily certified as medical devices«, as Prof. Hampe pointed out. Uwe Gäbler outlines more drastically: »Young patients are not waiting, informed parents are using every available device to help their children or to analyze their own condition with excellent wearables or apps - they are not waiting for the medical technology companies«. This is currently leading to a disruption in the market, as wearables are already widespread and some are even being used by doctors, even though they are not officially certified. The technical reality is turning the old order upside down and medtech OEMs need to adapt.
Despite this current lack of uniformity, all panel participants were confident about the long-term integration of Io(M)T devices in medicine and see huge market opportunities in the near future. Particularly in the area of consumables, »single use« will win out over nature and thus the fear of death. When asked whether patients should entrust their lives to technology and connectivity, NXP's Henning Möller said: »Automotive has also solved these problems, not so long ago, every steering wheel is controlled by microcontrollers these days. We can trust that this process will also work in medicine.« Nevertheless, there was agreement that standardized interfaces (such as surgical networks according to ISO standards) and stronger networking are necessary in order to increase the speed of innovation towards personalized medicine across the entire patient journey. (uh)









