Development of Medical Devices
Fast, faster, in-Silico Testing
Testing via computer model and simulation saves time and money. Thanks to in-silico testing, medical devices are initially developed, tested and validated without physical prototypes. Dr. Visa Suomi from Mathworks answered questions for our technical article and explains the advantages and hurdles.
The development and testing of medical devices has changed considerably in recent years with the introduction of in-silico testing. Although not new, this relatively new method uses computer-aided modeling and simulation and offers a virtual environment for the development, testing and validation of medical devices.
»In-silico testing means using computer modeling and simulation for the testing and development of medical devices or medical treatments. It's a virtual environment where developers would normally test a physical medical device and a physical patient. Now they do it in a virtual simulation«, explains Dr. Visa Suomi, Industry Manager Medical Devices at Mathworks.
Computer Models and Digital Twins
In-silico testing not only creates virtual models of the medical device, but also of the physiological environment in which it operates.
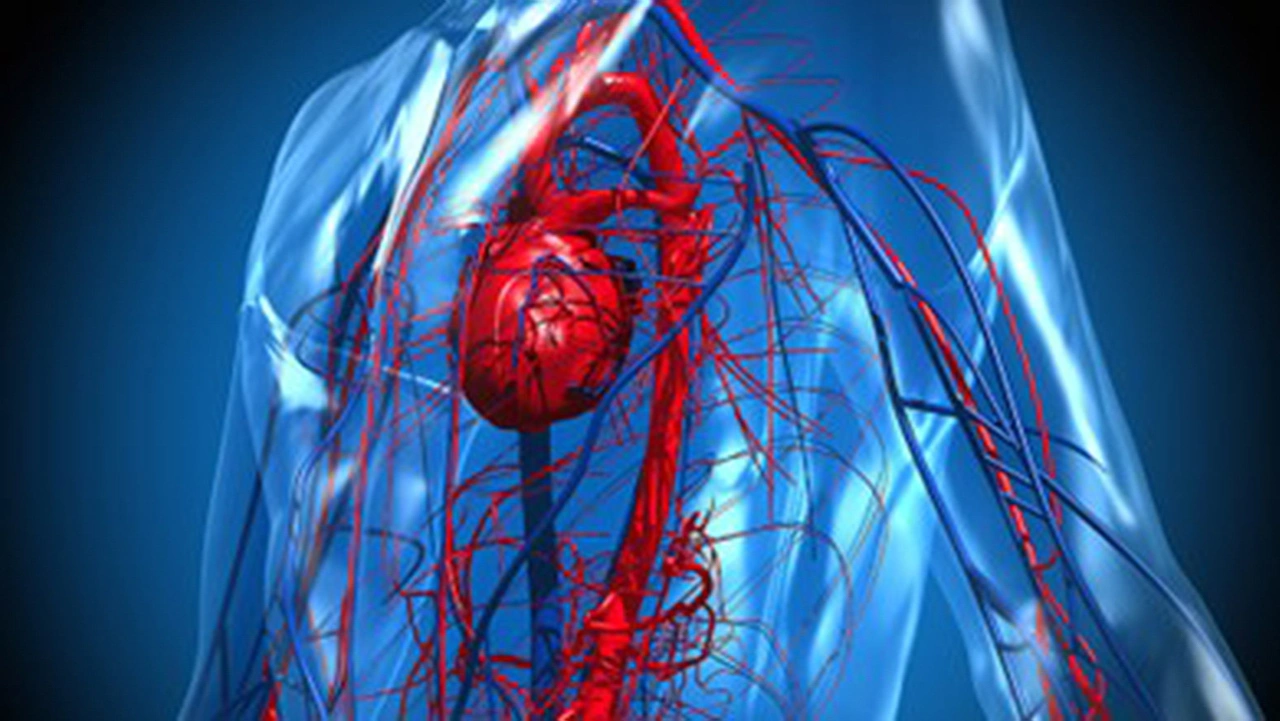
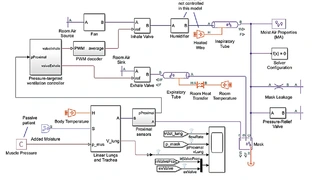
As virtual replicas of physical units such as medical devices or human organs, these models are de facto digital twins that use computer models to simulate the behavior and interaction of these units in a virtual environment (Figure 1). For example, the design of a ventilator, including all sensors, tubes and safety systems, can be simulated together with a virtual model of a patient's lungs.
The digital device twin maps the airflow, pressure and safety mechanisms, while the organ twin simulates the lung pressure, humidity and organic airflow. The behavior of the device is comprehensively tested under different conditions via the digital images of the medical device and human without the need to build physical prototypes or involve patients. The simulations can help to identify design flaws in advance and optimize the performance of the future medical device at a very early stage of the development process.

Advantages of in-Silico Testing
Herkömmliche Prüfansätze für Medizinprodukte basieren auf physischen Prototypen und langwierigen klinischen Studien, die nicht nur zeitaufwendig, sondern auch teuer sind. In-silico-Tests stellen dagegen eine vielversprechende Alternative dar, da sie eine frühzeitige Prüfung und Verfeinerung von Medizinprodukten in einer virtuellen Umgebung ermöglichen und so den Bedarf an umfangreichen physischen Prototypen und klinischen Studien verringern.
Dr. Visa Suomi bestätigt: »Als großer Vorteil des In-silico-Testings können Medtech-Entwickler das Verhalten ihres Medizinprodukts sehr früh simulieren und direkt an der Zielgruppe testen. Sie können unter vielfältigsten Bedingungen prüfen, wie sich die Funktionalität des Gerätes bei einem simulierten Patienten verhält, ohne dass dazu ein physischer Prototyp oder reale klinische Versuchsaufbauten notwendig wären. Das spart unheimlich viel Zeit und Geld.«
| Vorteile von In-silico-Tests | |
|---|---|
| Lower development costs | Reduced need for physical prototypes and clinical trials |
| Time saving | Early identification of design problems, accelerated development process |
| Security | Fewer risks from clinical trials on patients or animals, increased device safety |
| Flexibility | Easy modification and testing of various design parameters without building new prototypes |
One of the main advantages of in silico testing is the ability to simulate complex physiological behaviors and interactions. In the development of cardiovascular devices, for example, virtual models can simulate heart pressure, blood flow and electrical activity, providing a detailed understanding of how the device will interact with the human body.
The ability to model and evaluate a wide range of environmental conditions and case scenarios is particularly beneficial for devices with complicated control systems, such as surgical robots and infusion pumps, where accurate modeling of both the device and the physiological environment is critical to ensure safety and efficacy.
| Easy Start: Advice and Ready-Made Medtech Models |
|---|
|
In-silico testing often presents medical device manufacturers with initial hurdles. One major obstacle is the required expertise in the field of computer modeling and simulation, which is often still lacking, especially in small and medium-sized companies. Software manufacturers such as Mathworks, which offer programming platforms such as Matlab or simulation programs such as Simulink, therefore offer additional consulting for application engineers to support medical technology companies in the development and validation of their simulation models. In addition, ready-made models for various medical devices such as cardiovascular systems, ventilators, infusion pumps and dialysis machines are available for download. The models serve as a starting point for the creation of individual simulation setups and are intended to facilitate the introduction and launch of in-silico tests, particularly for SMEs. www.mathworks.com/medical |
In-silico-Entwicklung in der Praxis
Mathworks' reference customers who have already integrated in-silico testing into their development process include Siemens Healthineers' robotics subsidiary Corindus and diabetes specialist Tandem Diabetics Care. The medical robotics engineers at Corindus use in-silico testing to simulate the behavior of robotic arms and actuators in surgical robotic systems. This allows the design and functionality to be optimized before the robot developers tackle physical prototypes.
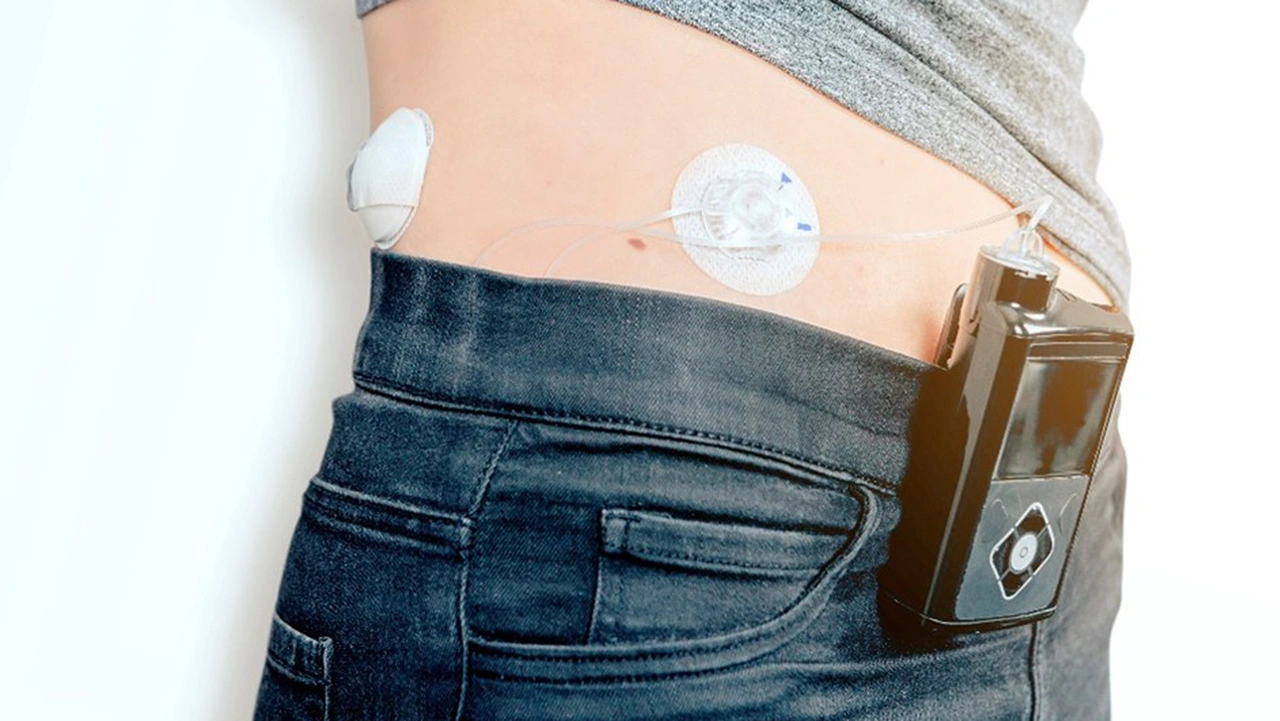
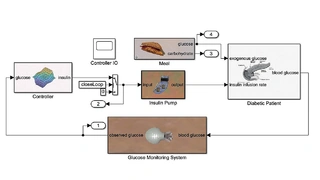
Tandem Diabetes Care uses in silico tests for the t:slim X2 artificial pancreas, which enables continuous glucose monitoring and insulin delivery (Figure 2). For an accelerated market launch, the behavior of the medical device was validated using both clinical data and in silico models (Figure 3).
Schnell, schneller, In-silico-Testing, Bilder 3-5
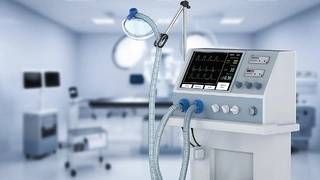
According to Dr. Suomi, in-silico tests delivered impressive results during the coronavirus pandemic: »At the height of COVID-19, the British government wanted to make new ventilators available quickly due to the high demand with the 'UK Ventilator Challenge'. Computer modeling and simulation were used to speed up the design process. This allowed the ventilator design to be checked before any hardware was built.« In just 47 days, the Cambridge Consultants team designed a simple new ventilator (Figures 4 and 5) that offers multiple settings for clinicians, eight alarms and a customized mix of air and oxygen.
Regulatorische Akzeptanz?
The advantages of virtual testing are obvious, but are initially of secondary importance in the medical context: The decisive factor for widespread use in medtech development is regulatory acceptance of in silico testing. The FDA (Food and Drug Administration) has been proactive in this regard, publishing guidelines and white papers outlining how computer modeling and simulation can be used in the review and validation of medical devices. In particular, the FDA has already accepted simulation results as evidence in certain cases, Dr. Suomi has an example: »In one case, the manufacturer of pacemakers used simulation to prove that the heat generated during an MRI scan was below the threshold accepted by the FDA.« The simulation and medical expert thus points to the increasing credibility of in silico tests in the regulatory landscape.
On adaptation in medical technology, Dr. Suomi says: »The American Medical Device Innovation Consortium (MDIC) conducts a survey every five years on the biggest challenges in the introduction of technologies. In the last two reports, medtech manufacturers mostly cited regulatory concerns and uncertainty as the biggest hurdles. We can now refute that: The FDA has already published reports and examples of companies using this method. And the EU has also included in-silico testing in its legislation, which means there is a secure regulatory basis.«
The European Medical Device Regulation (MDR) generally accepts in silico tests as part of the technical documentation for medical devices. As a condition, the models used must be validated and their accuracy proven. This means that the models must be reliable and reproducible in order to be accepted by the regulatory authorities. However, in silico tests alone are often not sufficient. For a CE mark, they need to be complemented by other data sources such as clinical studies or in vitro tests to ensure a comprehensive assessment of safety and efficacy. Manufacturers must therefore ensure that the in silico tests meet the specific requirements of the MDR, including regulatory compliant documentation and reporting of results.
Herausforderungen und Zukunft von In-silico-Tests
Although in silico tests offer numerous advantages, there are a number of other challenges to their widespread introduction in addition to regulatory issues:
- Model fidelity: the accuracy of in silico tests depends on the accuracy of their underlying computational models. High-fidelity models require extensive research and validation to ensure that they accurately replicate real-world behavior.
- Organizational hurdles: Smaller companies in particular may not have the in-house expertise required for computer-aided modeling and simulation. This requires intensive thematic training and knowledge acquisition.
- High initial costs: Although in-silico testing reduces long-term development costs, the initial investment for developing and validating computer models can be very high.
Despite the initial costs associated with the development and validation of simulation models, the long-term benefits of in-silico testing outweigh the costs. According to Dr. Suomi, medical technology companies in particular can significantly reduce their research and development costs and times by relying less on physical prototypes and more on virtual simulations. This approach also allows for continuous improvement and refinement of models, making the development process more efficient and adaptable to new technologies and requirements.
In-silico testing is therefore seen as having great potential to transform the development of medical devices, as it is a more efficient, cost-effective and safer alternative to traditional physical testing methods. As the calculation models continue to improve and the regulatory authorities increasingly accept the simulation results, it is to be expected that in silico tests will be used more and more frequently, which should lead to faster and more innovative development of medical devices, especially under the current market and technology pressure.





