New measuring method of the TU Vienna
Capacity increase of Li-ion batteries
Li-ion batteries have become an indispensable part of everyday life. Their performance is high, but can still be improved. Researchers at the TU Vienna have succeeded - but how?
Lithium-ion (Li-ion) batteries are found in smartphones, laptops and electric cars. But the possibilities of energy storage are far from exhausted. An idea to increase the capacity of the batteries: Replace graphite with silicon. However, a very thin boundary layer is needed to analyze and optimize the electrode, and there is currently no suitable investigation method for this.
Researchers at the TU Vienna and the University of Hasselt in Belgium have developed a technique with which the properties of the layers can be precisely measured. The whole thing works as follows: A force microscope exerts a precisely defined force, and at the same time light waves are used to register exactly how much the material is deformed as a result. Using the microscope, it is possible to search for suitable electrolytes with which the capacity of Li-ion batteries can be improved by a factor of up to six.
The number of ions is important
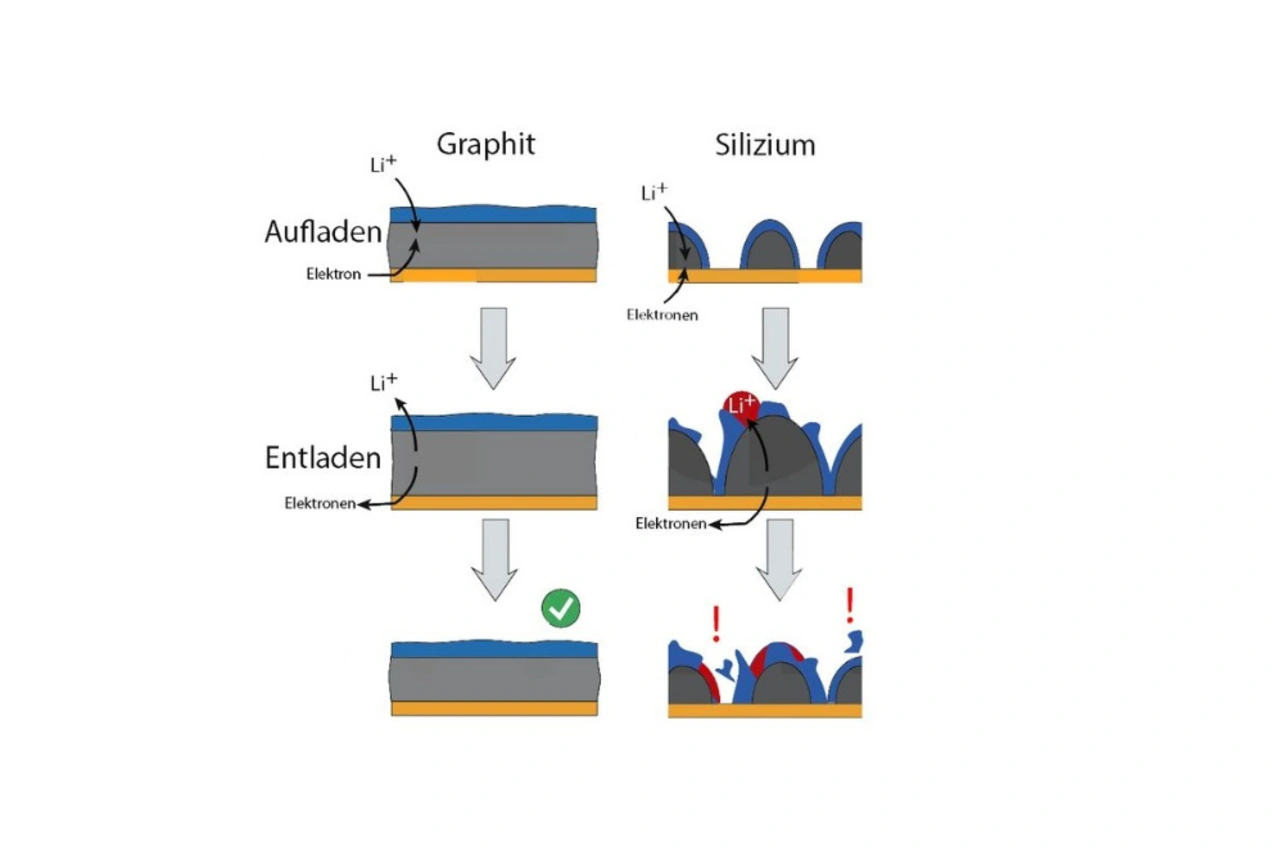
In a fully charged Li-ion battery the lithium ions are currently stored in graphite to stabilize electrons. While the battery is being charged, more and more ions and electrons migrate into the graphite and store electrical energy. In the graphite, six carbon atoms form a ring, in the middle of which an electron and a lithium ion can be held. However, small silicon crystals would be more suitable, says Markus Valtiner from the TU Vienna. »One lithium ion could be stored per silicon atom, theoretically increasing the storage capacity sixfold«.
The problem: In contrast to graphite, silicon granules in a battery are up to four times larger when lithium is absorbed and can simply crumble. One possibility is not to fully charge the silicon, but to produce another, more stable particle. However, the electrode granules in the battery are additionally coated with a nanometer-thin layer.
In a Li-ion battery, the materials are in constant contact with the liquid electrolyte, in which complex chemical degradation reactions take place during charging and discharging. A thin film of ion-conductive degradation products forms on the surface of the electrodes. On graphite, the layer is stable after a few charging cycles and no longer grows. In the case of silicon, however, the thin boundary layer tears open again and again and a new layer forms on the cracks. During each charging cycle this consumes a little of the electrolyte, which leads to a short battery life.
New measuring method leads to success
»The ideal solution would be an elastic boundary layer that would not crack if the silicon granule were to grow,« says Markus Valtiner. This requires the development of special electrolytes that make the layer elastic. The TU Vienna has developed a suitable measuring device for this purpose. Using a special atomic force microscope, the scientists can precisely analyze the elastic properties of the boundary layer – especially during charging and discharging. A special design allows the growth and elasticity of the boundary layer to be measured on a very small scale while a force is applied to it. Markus Valtiner's team intends to use the design to investigate different material variants and find suitable electrolytes for silicon-based lithium-ion batteries.
Markus Valtiner considers an increase in battery capacity by twice as much to be realistic. Initial results already show that silicon-based batteries can achieve 15 to 50 percent higher storage densities. The market launch of the new technology is expected in the next three to five years.






